Examples of Chemical Characteristics That Define Reactivity and Behavior Across Substances
Examples of Chemical Characteristics That Define Reactivity and Behavior Across Substances
From the explosive power of sulfuryl chloride to the life-sustaining stability of water, chemical characteristics determine how substances interact, transform, and behave in environments ranging from industrial labs to natural ecosystems. These intrinsic properties—dictated by atomic structure, bonding, and electron dynamics—nonetheless govern everything from combustion efficiency to biological compatibility. By examining key exemplars across diverse chemical classes, a clearer picture emerges of how chemical behavior is fundamentally predictable, yet uniquely nuanced.
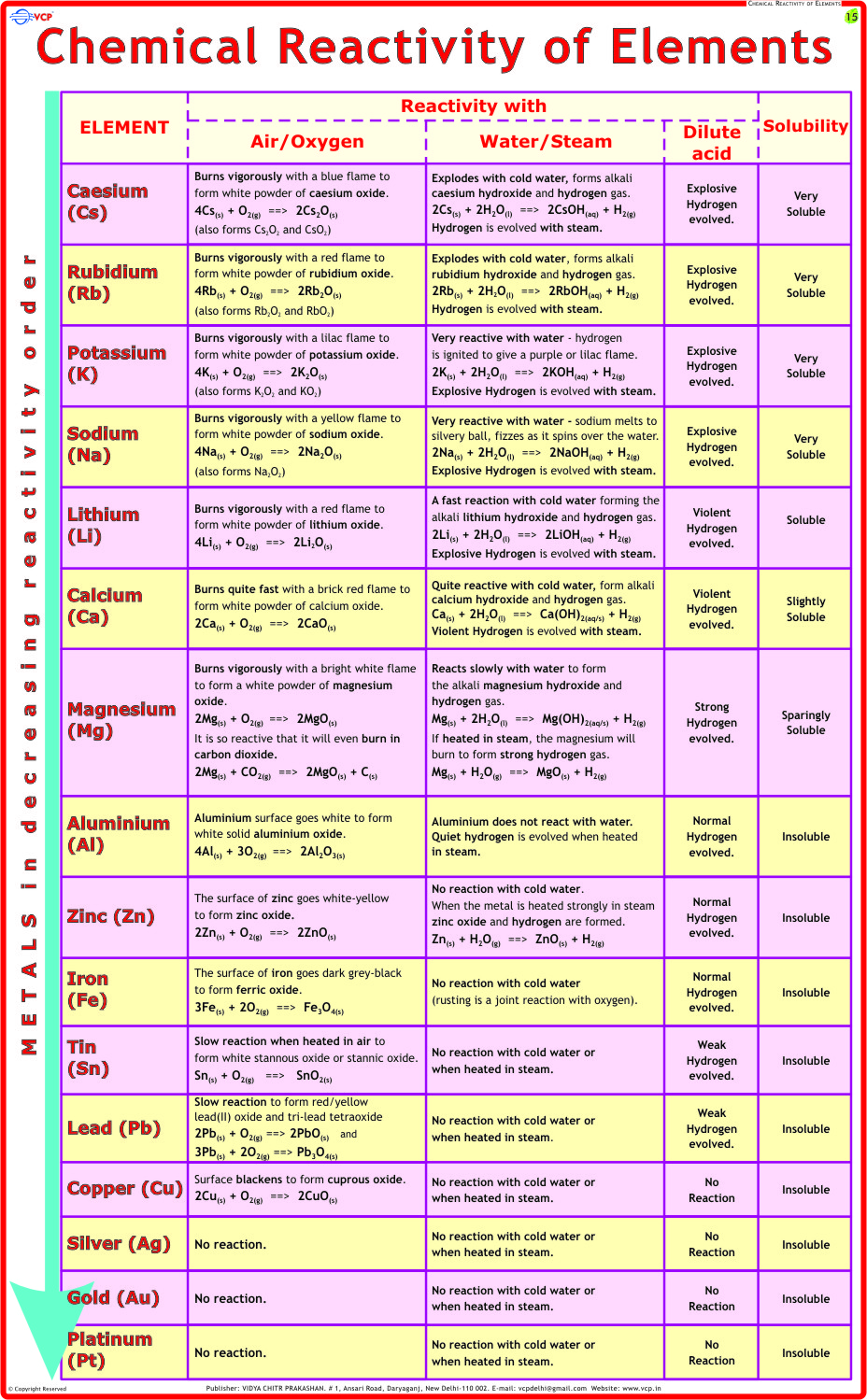
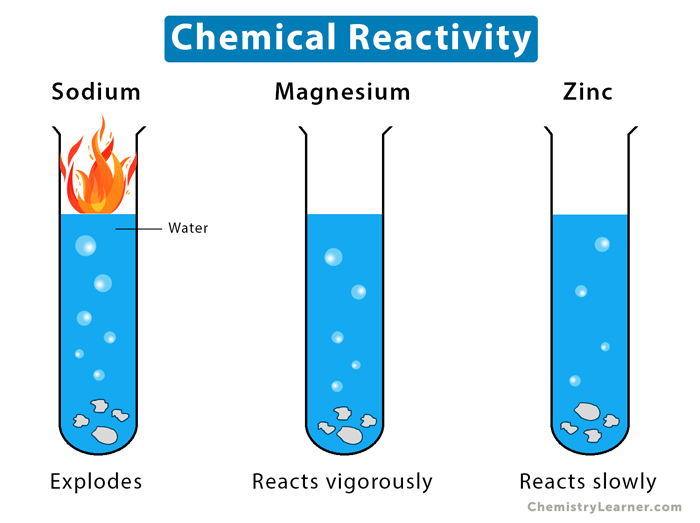
Among the most defined chemical characteristics is reactivity, which determines how readily a substance undergoes transformation. For instance, fluorine exemplifies extreme reactivity as the most electronegative element, readily oxidizing metals and even reacting exothermically with noble gases under certain conditions. Its vigorous interactions stem from a near-absolute electron-attraction tendency, making fluorine a cornerstone in polar synthesis and industrial fluorination.
As chemist Jane Jacobs notes, “Fluorine doesn’t just react—it demands reaction,” encapsulating its commanding role in chemical narratives. In contrast, chlorine, though still reactive, demonstrates controlled industrial behavior—critical in disinfectants and solvents. Intermolecular forces further shape material behavior. Water’s exceptional hydrogen

Related Post

Laura Ingraham’s Height & Weight Spotlight: What Public Figure Tells Us About Health, Image, and Public Perception

Samsung 2022 Shines Across Devices: From Flagship Phones to Visionary TVs

Meet the New 90 Day Fiancé Cast for 2025 — Love, Lottery, and Rising Influence

Camel Slang Explained: Decoding the Hidden Meanings Behind the Retail Mascot Language

